Learn More About the SYNVISC® Family
Rapid and long-lasting (up to 6 months) OA knee pain relief1-5
- As early as 1 week compared with baseline in a pivotal study of SYNVISC (57.7 vs 69.7 on VAS, P=0.0001), determined by patient assessment3,a
- Maintained at 6 months in pivotal studies of SYNVISC (35.7 vs 69.7 baseline on VAS, P=0.0001) and Synvisc-One® (1.43 vs 2.30 baseline on WOMAC A, P=0.047)3,4,a,b
- At 12 months, a 44.8% reduction in pain from baseline was maintained with SYNVISC in a randomized, controlled, clinical trial versus Hyalgan5,c
- Safety profiles of SYNVISC/Synvisc-One were similar to placebo1-4,a,b
EVIDENCE

In separate, head-to-head clinical trials, only the SYNVISC family of viscosupplements demonstrated superior OA knee pain relief versus all of the following: Saline Control1-4,a,b (P<0.05), Non-crosslinked sodium hyaluronate (Hyalgan)5,c (P=0.02), ICS (intra-articular corticosteroid) injection (triamcinolone hexacetonide)6,d.


- SYNVISC®/Synvisc-One® is the highest molecular weight HA available with a defined molecular weight1-2, 7-23,a
- Only SYNVISC/Synvisc-One is composed of 2 unique formulations of crosslinked polymers, a fluid and a gel.15-19
- SYNVISC®/Synvisc-One® is designed to stay in the knee joint longer than non-crosslinked viscosupplements1-2, 24-25,b
- Crosslinking results in longer intra-articular residence times for SYNVISC/Synvisc-One (58 and 44 days, respectively, compared with <72 hours for unmodified hyaluronic acid products).26-27,b
a Among currently approved viscosupplements with a defined molecular weight.
The clinical significance of these physical properties is unknown. Comparisons cannot be made in absence of head-to-head clinical trials. This information is not based on any head-to-head clinical trial.

The SYNVISC family of viscosupplements has an established safety profile.5,6,9 81% patient satisfaction in a large observational clinical study to assess tolerability of SYNVISC28
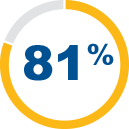
of patients were satisfied with SYNVISC as their therapy, rating it as "good" or "very good", at 3 weeks after the first injection1
Tolerability was assessed by documenting the incidence and type of local AEs as well as severity and relation to therapy. Treatment AEs were reported in 4.2% of patients (2.4% of injections) and most were mild (21.4%) to moderate (40.3%)
EXPERIENCE

SYNVISC and Synvisc-One have been included in more than 300 publications and used to treat more than 20 million knees worldwide26
Resources for Your Patients
MAT-US-2106837-v1.0-07/2021
Indication
SYNVISC® (hylan G-F 20) and SYNVISC-ONE® (hylan G-F 20) are indicated for the treatment of pain in osteoarthritis (OA) of the knee in patients who have failed to respond adequately to conservative nonpharmacologic therapy and simple analgesics, e.g., acetaminophen.
IMPORTANT SAFETY INFORMATION
Important Safety Information
SYNVISC and SYNVISC-ONE are contraindicated in patients with known hypersensitivity to hyaluronan products or patients with infections in or around the target knee. Hypersensitivity reactions including anaphylactic reaction, anaphylactoid reaction, anaphylactic shock and angioedema have been reported for both SYNVISC and SYNVISC-ONE.
Do not concomitantly use disinfectants containing quaternary ammonium salts for skin preparation because hyaluronan can precipitate in their presence. Do not inject SYNVISC or SYNVISC-ONE extra-articularly, into the synovial tissues, into the fat pad or joint capsule, or intravascularly. Some cases of skin necrosis have been reported after intra-articular use of hyaluronic acid. Patients should be instructed to contact their treating physician if signs of skin disorder (such as change of color or open sores) appear.
The safety and efficacy of SYNVISC-ONE in locations other than the knee, or for conditions other than osteoarthritis, or in combination with other intra-articular injectables, or in severely inflamed knee joints have not been established. Use caution when injecting SYNVISC or SYNVISC-ONE in patients allergic to avian proteins, feathers, or egg products; who have evidence of lymphatic or venous stasis in the leg to be treated; or who have severe inflammation in the knee to be treated. Remove any synovial fluid or effusion before injecting SYNVISC or SYNVISC-ONE. Strict adherence to aseptic technique must be followed to avoid joint infection. The safety and effectiveness of SYNVISC and SYNVISC-ONE have not been established in children (≤21 years old) or in pregnant or lactating women. Patients should be advised to avoid strenuous or prolonged weight-bearing activities for approximately 48 hours after treatment.
For SYNVISC
In clinical trials, the most commonly reported adverse events were transient pain, swelling, and/or joint effusion in the injected knee. The following reported adverse events are among those that may occur in association with intra-articular injections, including SYNVISC: arthralgia, joint stiffness, joint effusion, joint swelling, joint warmth, injection site pain, arthritis, arthropathy, and gait disturbance.
View the Complete Prescribing Information for SYNVISC
For SYNVISC-ONE
The most commonly reported adverse events were arthralgia, arthritis, arthropathy, injection site pain, and joint effusion. The following reported adverse events are among those that may occur in association with intra-articular injections, including SYNVISC-ONE: arthralgia, joint stiffness, joint effusion, joint swelling, joint warmth, injection site pain, arthritis, arthropathy, and gait disturbance.
View the Complete Prescribing Information for SYNVISC-ONE
References
- SYNVISC [prescribing information]. Cambridge, MA: Genzyme Corporation; 2014.
- Synvisc-One [prescribing information]. Cambridge, MA: Genzyme Corporation; 2014.
- Wobig M, Dickhut A, Maier R, Vetter G. Viscosupplementation with hylan G-F 20: a 26-week controlled trial of efficacy and safety in the osteoarthritic knee. Clin Ther. 1998;20(3):410-423.
- Chevalier X, Jerosch J, Goupille P, et al. Single, intra-articular treatment with 6 ml hylan G-F 20 in patients with symptomatic primary osteoarthritis of the knee: a randomised, multicentre, double-blind, placebo controlled trial. Ann Rheum Dis. 2010;69(1):113-119.
- Raman R, Dutta A, Day N, Sharma HK, Shaw CJ, Johnson GV. Efficacy of hylan G-F 20 and sodium hyaluronate in the treatment of osteoarthritis of the knee—a prospective randomized clinical trial. Knee. 2008;15(4):318-324.
- Caborn D, Rush J, Lanzer W, Parenti D, Murray C. A randomized, single-blind comparison of the efficacy and tolerability of hylan G-F 20 and triamcinolone hexacetonide in patients with osteoarthritis of the knee. J Rheumatol. 2004;31(2):333-343.
- Gel-One [prescribing information]. Warsaw, IN: Zimmer USA; 2011.
- Monovisc summary of safety and effectiveness data. Silver Spring, MD: US Food and Drug Administration; 2014.
- Euflexxa [prescribing information]. Parsippany, NJ: Ferring Pharmaceuticals; 2016.
- Orthovisc [prescribing information]. Raynham, MA: DePuy Mitek Inc; 2005.
- Hyalgan [prescribing information]. Parsippany, NJ: Fidia Pharma USA Inc; 2014.
- Supartz FX [prescribing information]. Durham, NC: Bioventus LLC; 2015.
- Hymovis [prescribing information]. Parsippany, NJ: Fidia Pharma USA Inc; 2015.
- GenVisc 850 [prescribing information]. Doylestown, PA: OrthogenRx Inc; 2015.
- Gelsyn-3 [prescribing information]. Pambio-Noranco, Switzerland: Institut Biochimique SA; 2016.
- Visco-3 [prescribing information]. Durham, NC: Bioventus LLC; 2016.
- Ågerup B, Berg P, Åkermark C. Non-animal stabilized hyaluronic acid: a new formulation for the treatment of osteoarthritis. BioDrugs. 2005;19(1):23-30.
- TriVisc [prescribing information]. Doylestown, PA: OrthogenRx Inc; 2017.
- Balazs EA, Watson D, Duff IF, Roseman S. Hyaluronic acid in synovial fluid. I. Molecular parameters of hyaluronic acid in normal and arthritic human fluids. Arthritis Rheum. 1967;10(4):357-376.
- Larsen NE, Dursema HD, Pollak CT, Skrabut EM. Clearance kinetics of a hylan-based viscosupplement after intra-articular and intravenous administration in animal models. J Biomed Mater Res B Appl Biomater. 2012;100(2):457-462.
- Balazs EA, Leshchiner A, Inventors; Biomatrix, Inc, Ridgefield, NJ, assignee. Cross-linked gels of hyaluronic acid and products containing such gels. US patent 4,582,865. April 15, 1986.
- Balazs EA, Leshchiner A, Leshchiner A, Band P, Inventors; Biomatrix, Inc, Ridgefield, NJ, assignee. Chemically modified hyaluronic acid preparation and method of recovery thereof from animal tissues. US patent 4,713,448. December 15, 1987.
- Sanofi. The Making of SYNVISC [video]. 2001.
- Bhuanantanondh P, Grecov D, Kwok E. Rheological study of viscosupplements and synovial fluid in patients with osteoarthritis. J Med Biol Eng. 2012;32(1):12-16.
- Henrotin Y, Raman R, Richette P, et al. Consensus statement on viscosupplementation with hyaluronic acid for the management of osteoarthritis. Semin Arthritis Rheum. 2015;45(2):140-149
- Data on file. Genzyme Corporation.
- Larsen NE, Dursema HD, Pollak CT, Skrabut EM. Clearance kinetics of a hylan-based viscosupplement after intra-articular and intravenous administration in animal models. J Biomed Mater Res B Appl Biomater. 2012;100(2):457-462.
- Kemper F, Gebhardt U, Meng T, Murray C. Tolerability and short-term effectiveness of hylan G-F 20 in 4253 patients with osteoarthritis of the knee in clinical practice. Curr Med Res Opin. 2005;21(8):1261-1269.
IMPORTANT SAFETY INFORMATION
Read MoreImportant Safety Information
SYNVISC and SYNVISC-ONE are contraindicated in patients with known hypersensitivity to hyaluronan products or patients with infections in or around the target knee. Hypersensitivity reactions including anaphylactic reaction, anaphylactoid reaction, anaphylactic shock and angioedema have been reported for both SYNVISC and SYNVISC-ONE.
Do not concomitantly use disinfectants containing quaternary ammonium salts for skin preparation because hyaluronan can precipitate in their presence. Do not inject SYNVISC or SYNVISC-ONE extra-articularly, into the synovial tissues, into the fat pad or joint capsule, or intravascularly. Some cases of skin necrosis have been reported after intra-articular use of hyaluronic acid. Patients should be instructed to contact their treating physician if signs of skin disorder (such as change of color or open sores) appear.
The safety and efficacy of SYNVISC-ONE in locations other than the knee, or for conditions other than osteoarthritis, or in combination with other intra-articular injectables, or in severely inflamed knee joints have not been established. Use caution when injecting SYNVISC or SYNVISC-ONE in patients allergic to avian proteins, feathers, or egg products; who have evidence of lymphatic or venous stasis in the leg to be treated; or who have severe inflammation in the knee to be treated. Remove any synovial fluid or effusion before injecting SYNVISC or SYNVISC-ONE. Strict adherence to aseptic technique must be followed to avoid joint infection. The safety and effectiveness of SYNVISC and SYNVISC-ONE have not been established in children (≤21 years old) or in pregnant or lactating women. Patients should be advised to avoid strenuous or prolonged weight-bearing activities for approximately 48 hours after treatment.
For SYNVISC
In clinical trials, the most commonly reported adverse events were transient pain, swelling, and/or joint effusion in the injected knee. The following reported adverse events are among those that may occur in association with intra-articular injections, including SYNVISC: arthralgia, joint stiffness, joint effusion, joint swelling, joint warmth, injection site pain, arthritis, arthropathy, and gait disturbance.
View the Complete Prescribing Information for SYNVISC
For SYNVISC-ONE
The most commonly reported adverse events were arthralgia, arthritis, arthropathy, injection site pain, and joint effusion. The following reported adverse events are among those that may occur in association with intra-articular injections, including SYNVISC-ONE: arthralgia, joint stiffness, joint effusion, joint swelling, joint warmth, injection site pain, arthritis, arthropathy, and gait disturbance.
View the Complete Prescribing Information for SYNVISC-ONE
References
- SYNVISC [prescribing information]. Cambridge, MA: Genzyme Corporation; 2014.
- Synvisc-One [prescribing information]. Cambridge, MA: Genzyme Corporation; 2014.
- Wobig M, Dickhut A, Maier R, Vetter G. Viscosupplementation with hylan G-F 20: a 26-week controlled trial of efficacy and safety in the osteoarthritic knee. Clin Ther. 1998;20(3):410-423.
- Chevalier X, Jerosch J, Goupille P, et al. Single, intra-articular treatment with 6 ml hylan G-F 20 in patients with symptomatic primary osteoarthritis of the knee: a randomised, multicentre, double-blind, placebo controlled trial. Ann Rheum Dis. 2010;69(1):113-119.
- Raman R, Dutta A, Day N, Sharma HK, Shaw CJ, Johnson GV. Efficacy of hylan G-F 20 and sodium hyaluronate in the treatment of osteoarthritis of the knee—a prospective randomized clinical trial. Knee. 2008;15(4):318-324.
- Caborn D, Rush J, Lanzer W, Parenti D, Murray C. A randomized, single-blind comparison of the efficacy and tolerability of hylan G-F 20 and triamcinolone hexacetonide in patients with osteoarthritis of the knee. J Rheumatol. 2004;31(2):333-343.
- Gel-One [prescribing information]. Warsaw, IN: Zimmer USA; 2011.
- Monovisc summary of safety and effectiveness data. Silver Spring, MD: US Food and Drug Administration; 2014.
- Euflexxa [prescribing information]. Parsippany, NJ: Ferring Pharmaceuticals; 2016.
- Orthovisc [prescribing information]. Raynham, MA: DePuy Mitek Inc; 2005.
- Hyalgan [prescribing information]. Parsippany, NJ: Fidia Pharma USA Inc; 2014.
- Supartz FX [prescribing information]. Durham, NC: Bioventus LLC; 2015.
- Hymovis [prescribing information]. Parsippany, NJ: Fidia Pharma USA Inc; 2015.
- GenVisc 850 [prescribing information]. Doylestown, PA: OrthogenRx Inc; 2015.
- Gelsyn-3 [prescribing information]. Pambio-Noranco, Switzerland: Institut Biochimique SA; 2016.
- Visco-3 [prescribing information]. Durham, NC: Bioventus LLC; 2016.
- Ågerup B, Berg P, Åkermark C. Non-animal stabilized hyaluronic acid: a new formulation for the treatment of osteoarthritis. BioDrugs. 2005;19(1):23-30.
- TriVisc [prescribing information]. Doylestown, PA: OrthogenRx Inc; 2017.
- Balazs EA, Watson D, Duff IF, Roseman S. Hyaluronic acid in synovial fluid. I. Molecular parameters of hyaluronic acid in normal and arthritic human fluids. Arthritis Rheum. 1967;10(4):357-376.
- Larsen NE, Dursema HD, Pollak CT, Skrabut EM. Clearance kinetics of a hylan-based viscosupplement after intra-articular and intravenous administration in animal models. J Biomed Mater Res B Appl Biomater. 2012;100(2):457-462.
- Balazs EA, Leshchiner A, Inventors; Biomatrix, Inc, Ridgefield, NJ, assignee. Cross-linked gels of hyaluronic acid and products containing such gels. US patent 4,582,865. April 15, 1986.
- Balazs EA, Leshchiner A, Leshchiner A, Band P, Inventors; Biomatrix, Inc, Ridgefield, NJ, assignee. Chemically modified hyaluronic acid preparation and method of recovery thereof from animal tissues. US patent 4,713,448. December 15, 1987.
- Sanofi. The Making of SYNVISC [video]. 2001.
- Bhuanantanondh P, Grecov D, Kwok E. Rheological study of viscosupplements and synovial fluid in patients with osteoarthritis. J Med Biol Eng. 2012;32(1):12-16.
- Henrotin Y, Raman R, Richette P, et al. Consensus statement on viscosupplementation with hyaluronic acid for the management of osteoarthritis. Semin Arthritis Rheum. 2015;45(2):140-149
- Data on file. Genzyme Corporation.
- Larsen NE, Dursema HD, Pollak CT, Skrabut EM. Clearance kinetics of a hylan-based viscosupplement after intra-articular and intravenous administration in animal models. J Biomed Mater Res B Appl Biomater. 2012;100(2):457-462.
- Kemper F, Gebhardt U, Meng T, Murray C. Tolerability and short-term effectiveness of hylan G-F 20 in 4253 patients with osteoarthritis of the knee in clinical practice. Curr Med Res Opin. 2005;21(8):1261-1269.

