Science Behind SYNVISC® Family
SYNVISC®/Synvisc-One® is specifically designed to match the properties of
healthy, young synovial fluid1-3
Physical properties of synovial fluid:
| weight (MDa)* | Elasticity (Pa at 2.5 Hz)* |
Viscosity (Pa at 2.5 Hz)* |
|
|---|---|---|---|
| Healthy, young synovial fluid1-3 |
6† |
117‡ |
45‡ |
| Osteoarthritic synovial fluid4-6 |
1.6 |
1.9 |
1.4 |
Physical properties of viscosupplements:
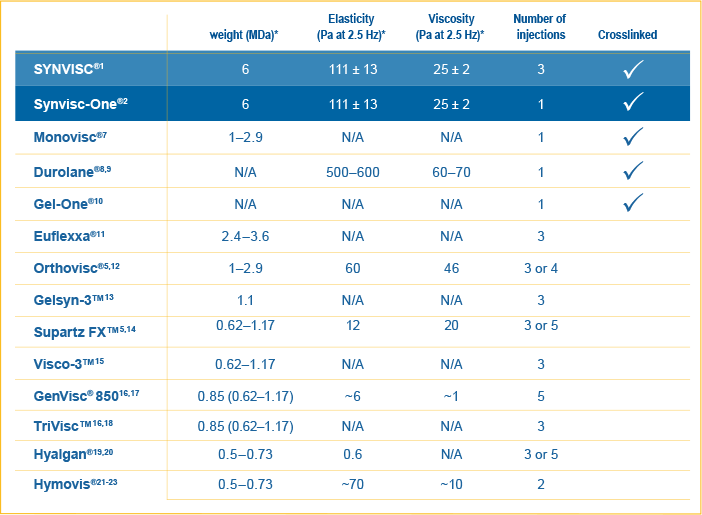
*The clinical significance of these physical properties is unknown. No comparative conclusions regarding safety or efficacy should be derived from these data points.
†As in 21- to 45-year-old adults.
‡As in 21- to 45-year-old adults.
SYNVISC®/Synvisc-One® is designed to stay in the knee joint longer than non-crosslinked viscosupplements1-2, 24-25
Crosslinking results in longer intra-articular residence times for SYNVISC/Synvisc-One (58 and 44 days, respectively, compared with <72 hours for unmodified hyaluronic acid products).26-27,a
Crosslinking is important for HA because it provides24-25:
- Longer intra-articular residence time
- Higher molecular weight
- Increased lubricating and shock-absorbing properties
aThe clinical significance of these physical properties is unknown. This information is not based on any head-to-head clinical trial. Comparisons cannot be made in absence of head-to-head clinical trials.
SYNVISC®/Synvisc-One® is the highest molecular weight HA available with a defined molecular weight1-2,7-16,18,a
ONLY SYNVISC/Synvisc-One are composed of 2 unique formulations of crosslinked polymers, a fluid and a gel.3, 27,29-31
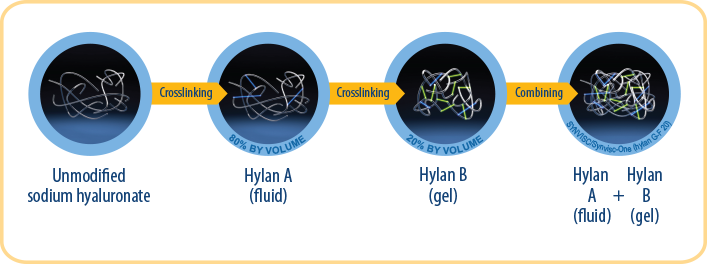
*The clinical significance of these physical properties is unknown.
SYNVISC/Synvisc-One is derived from a natural, avian source to achieve high molecular weight (6 MDa)1,2,28
- HA derived from bacterial production have molecular weights of 4 MDa or less28
Resources for Your Patients
MAT-US-2106837-v1.0-07/2021
Indication
SYNVISC® (hylan G-F 20) and SYNVISC-ONE® (hylan G-F 20) are indicated for the treatment of pain in osteoarthritis (OA) of the knee in patients who have failed to respond adequately to conservative nonpharmacologic therapy and simple analgesics, e.g., acetaminophen.
IMPORTANT SAFETY INFORMATION
Important Safety Information
SYNVISC and SYNVISC-ONE are contraindicated in patients with known hypersensitivity to hyaluronan products or patients with infections in or around the target knee. Hypersensitivity reactions including anaphylactic reaction, anaphylactoid reaction, anaphylactic shock and angioedema have been reported for both SYNVISC and SYNVISC-ONE.
Do not concomitantly use disinfectants containing quaternary ammonium salts for skin preparation because hyaluronan can precipitate in their presence. Do not inject SYNVISC or SYNVISC-ONE extra-articularly, into the synovial tissues, into the fat pad or joint capsule, or intravascularly. Some cases of skin necrosis have been reported after intra-articular use of hyaluronic acid. Patients should be instructed to contact their treating physician if signs of skin disorder (such as change of color or open sores) appear.
The safety and efficacy of SYNVISC-ONE in locations other than the knee, or for conditions other than osteoarthritis, or in combination with other intra-articular injectables, or in severely inflamed knee joints have not been established. Use caution when injecting SYNVISC or SYNVISC-ONE in patients allergic to avian proteins, feathers, or egg products; who have evidence of lymphatic or venous stasis in the leg to be treated; or who have severe inflammation in the knee to be treated. Remove any synovial fluid or effusion before injecting SYNVISC or SYNVISC-ONE. Strict adherence to aseptic technique must be followed to avoid joint infection. The safety and effectiveness of SYNVISC and SYNVISC-ONE have not been established in children (≤21 years old) or in pregnant or lactating women. Patients should be advised to avoid strenuous or prolonged weight-bearing activities for approximately 48 hours after treatment.
For SYNVISC
In clinical trials, the most commonly reported adverse events were transient pain, swelling, and/or joint effusion in the injected knee. The following reported adverse events are among those that may occur in association with intra-articular injections, including SYNVISC: arthralgia, joint stiffness, joint effusion, joint swelling, joint warmth, injection site pain, arthritis, arthropathy, and gait disturbance.
View the Complete Prescribing Information for SYNVISC
For SYNVISC-ONE
The most commonly reported adverse events were arthralgia, arthritis, arthropathy, injection site pain, and joint effusion. The following reported adverse events are among those that may occur in association with intra-articular injections, including SYNVISC-ONE: arthralgia, joint stiffness, joint effusion, joint swelling, joint warmth, injection site pain, arthritis, arthropathy, and gait disturbance.
View the Complete Prescribing Information for SYNVISC-ONE
References
- SYNVISC [prescribing information]. Cambridge, MA: Genzyme Corporation; 2014.
- Synvisc-One [prescribing information]. Cambridge, MA: Genzyme Corporation; 2014.
- Balazs EA, Watson D, Duff IF, Roseman S. Hyaluronic acid in synovial fluid. I. Molecular parameters of hyaluronic acid in normal and arthritic human fluids. Arthritis Rheum. 1967;10(4):357-376.
- Praest BM, Greiling H, Kock R. Assay of synovial fluid parameters: hyaluronan concentration as a potential marker for joint diseases. Clin Chim Acta. 1997;266(2):117-128.
- Mazzucco D, McKinley G, Scott RD, Spector M. Rheology of joint fluid in total knee arthroplasty patients. J Orthop Res. 2002;20(6):1157-1163.
- Watterson JR, Esdaile JM. Viscosupplementation: therapeutic mechanisms and clinical potential in osteoarthritis of the knee. J Am Acad Orthop Surg. 2000;8(5):277-284.
- Monovisc summary of safety and effectiveness data. Silver Spring, MD: US Food and Drug Administration; 2014.
- Ågerup B, Berg P, Åkermark C. Non-animal stabilized hyaluronic acid: a new formulation for the treatment of osteoarthritis. BioDrugs. 2005;19(1):23-30.
- Durolane [instructions for use]. Durham, NC: Bioventus LLC; 2017.
- Gel-One [prescribing information]. Warsaw, IN: Zimmer USA; 2011.
- Euflexxa [prescribing information]. Parsippany, NJ: Ferring Pharmaceuticals; 2016.
- Orthovisc [prescribing information]. Raynham, MA: DePuy Mitek Inc; 2005.
- Gelsyn-3 [prescribing information]. Pambio-Noranco, Switzerland: Institut Biochimique SA; 2016.
- Supartz FX [prescribing information]. Durham, NC: Bioventus LLC; 2015.
- Visco-3 [prescribing information]. Durham, NC: Bioventus LLC; 2016.
- GenVisc 850 [prescribing information]. Doylestown, PA: OrthogenRx Inc; 2015.
- Braithwaite GJ, Daley MJ, Toledo-Velasquez D. Rheological and molecular weight comparisons of approved hyaluronic acid products - preliminary standards for establishing class III medical device equivalence. J Biomater Sci Polym Ed. 2016;27(3):235-246.
- TriVisc [prescribing information]. Doylestown, PA: OrthogenRx Inc; 2017.
- Hyalgan [prescribing information]. Parsippany, NJ: Fidia Pharma USA Inc; 2014.
- Gomis A, Pawlak M, Balazs EA, Schmidt RF, Belmonte C. Effects of different molecular weight elastoviscous hyaluronan solutions on articular nociceptive afferents. Arthritis Rheum. 2004;50(1):314-326.
- Hymovis [prescribing information]. Parsippany, NJ: Fidia Pharma USA Inc; 2015.
- Smith MM, Russell AK, Schiavinato A, Little CB. A hexadecylamide derivative of hyaluronan (HYMOVIS®) has superior beneficial effects on human osteoarthritic chondrocytes and synoviocytes than unmodified hyaluronan. J Inflamm (Lond). 2013;10:26.
- Finelli I, Chiessi E, Galesso D, Renier D, Paradossi G. A new viscosupplement based on partially hydrophobic hyaluronic acid: a comparative study. Biorheology. 2011;48(5):263-275.
- Bhuanantanondh P, Grecov D, Kwok E. Rheological study of viscosupplements and synovial fluid in patients with osteoarthritis. J Med Biol Eng. 2012;32(1):12-16.
- Henrotin Y, Raman R, Richette P, et al. Consensus statement on viscosupplementation with hyaluronic acid for the management of osteoarthritis. Semin Arthritis Rheum. 2015;45(2):140-149.
- Data on file. Genzyme Corporation.
- Larsen NE, Dursema HD, Pollak CT, Skrabut EM. Clearance kinetics of a hylan-based viscosupplement after intra-articular and intravenous administration in animal models. J Biomed Mater Res B Appl Biomater. 2012;100(2):457-462.
- Boeriu CG, Springer J, Kooy FK, van den Broek LAM, Eggink G. Production methods for hyaluronan. Int J Carbohydr Chem. 2013;2013:1-14.
- Balazs EA, Leshchiner A, Inventors; Biomatrix, Inc, Ridgefield, NJ, assignee. Cross-linked gels of hyaluronic acid and products containing such gels. US patent 4,582,865. April 15, 1986.
- Balazs EA, Leshchiner A, Leshchiner A, Band P, Inventors; Biomatrix, Inc, Ridgefield, NJ, assignee. Chemically modified hyaluronic acid preparation and method of recovery thereof from animal tissues. US patent 4,713,448. December 15, 1987.
- Sanofi. The Making of SYNVISC [video]. 2001.
IMPORTANT SAFETY INFORMATION
Read MoreImportant Safety Information
SYNVISC and SYNVISC-ONE are contraindicated in patients with known hypersensitivity to hyaluronan products or patients with infections in or around the target knee. Hypersensitivity reactions including anaphylactic reaction, anaphylactoid reaction, anaphylactic shock and angioedema have been reported for both SYNVISC and SYNVISC-ONE.
Do not concomitantly use disinfectants containing quaternary ammonium salts for skin preparation because hyaluronan can precipitate in their presence. Do not inject SYNVISC or SYNVISC-ONE extra-articularly, into the synovial tissues, into the fat pad or joint capsule, or intravascularly. Some cases of skin necrosis have been reported after intra-articular use of hyaluronic acid. Patients should be instructed to contact their treating physician if signs of skin disorder (such as change of color or open sores) appear.
The safety and efficacy of SYNVISC-ONE in locations other than the knee, or for conditions other than osteoarthritis, or in combination with other intra-articular injectables, or in severely inflamed knee joints have not been established. Use caution when injecting SYNVISC or SYNVISC-ONE in patients allergic to avian proteins, feathers, or egg products; who have evidence of lymphatic or venous stasis in the leg to be treated; or who have severe inflammation in the knee to be treated. Remove any synovial fluid or effusion before injecting SYNVISC or SYNVISC-ONE. Strict adherence to aseptic technique must be followed to avoid joint infection. The safety and effectiveness of SYNVISC and SYNVISC-ONE have not been established in children (≤21 years old) or in pregnant or lactating women. Patients should be advised to avoid strenuous or prolonged weight-bearing activities for approximately 48 hours after treatment.
For SYNVISC
In clinical trials, the most commonly reported adverse events were transient pain, swelling, and/or joint effusion in the injected knee. The following reported adverse events are among those that may occur in association with intra-articular injections, including SYNVISC: arthralgia, joint stiffness, joint effusion, joint swelling, joint warmth, injection site pain, arthritis, arthropathy, and gait disturbance.
View the Complete Prescribing Information for SYNVISC
For SYNVISC-ONE
The most commonly reported adverse events were arthralgia, arthritis, arthropathy, injection site pain, and joint effusion. The following reported adverse events are among those that may occur in association with intra-articular injections, including SYNVISC-ONE: arthralgia, joint stiffness, joint effusion, joint swelling, joint warmth, injection site pain, arthritis, arthropathy, and gait disturbance.
View the Complete Prescribing Information for SYNVISC-ONE
References
- SYNVISC [prescribing information]. Cambridge, MA: Genzyme Corporation; 2014.
- Synvisc-One [prescribing information]. Cambridge, MA: Genzyme Corporation; 2014.
- Balazs EA, Watson D, Duff IF, Roseman S. Hyaluronic acid in synovial fluid. I. Molecular parameters of hyaluronic acid in normal and arthritic human fluids. Arthritis Rheum. 1967;10(4):357-376.
- Praest BM, Greiling H, Kock R. Assay of synovial fluid parameters: hyaluronan concentration as a potential marker for joint diseases. Clin Chim Acta. 1997;266(2):117-128.
- Mazzucco D, McKinley G, Scott RD, Spector M. Rheology of joint fluid in total knee arthroplasty patients. J Orthop Res. 2002;20(6):1157-1163.
- Watterson JR, Esdaile JM. Viscosupplementation: therapeutic mechanisms and clinical potential in osteoarthritis of the knee. J Am Acad Orthop Surg. 2000;8(5):277-284.
- Monovisc summary of safety and effectiveness data. Silver Spring, MD: US Food and Drug Administration; 2014.
- Ågerup B, Berg P, Åkermark C. Non-animal stabilized hyaluronic acid: a new formulation for the treatment of osteoarthritis. BioDrugs. 2005;19(1):23-30.
- Durolane [instructions for use]. Durham, NC: Bioventus LLC; 2017.
- Gel-One [prescribing information]. Warsaw, IN: Zimmer USA; 2011.
- Euflexxa [prescribing information]. Parsippany, NJ: Ferring Pharmaceuticals; 2016.
- Orthovisc [prescribing information]. Raynham, MA: DePuy Mitek Inc; 2005.
- Gelsyn-3 [prescribing information]. Pambio-Noranco, Switzerland: Institut Biochimique SA; 2016.
- Supartz FX [prescribing information]. Durham, NC: Bioventus LLC; 2015.
- Visco-3 [prescribing information]. Durham, NC: Bioventus LLC; 2016.
- GenVisc 850 [prescribing information]. Doylestown, PA: OrthogenRx Inc; 2015.
- Braithwaite GJ, Daley MJ, Toledo-Velasquez D. Rheological and molecular weight comparisons of approved hyaluronic acid products - preliminary standards for establishing class III medical device equivalence. J Biomater Sci Polym Ed. 2016;27(3):235-246.
- TriVisc [prescribing information]. Doylestown, PA: OrthogenRx Inc; 2017.
- Hyalgan [prescribing information]. Parsippany, NJ: Fidia Pharma USA Inc; 2014.
- Gomis A, Pawlak M, Balazs EA, Schmidt RF, Belmonte C. Effects of different molecular weight elastoviscous hyaluronan solutions on articular nociceptive afferents. Arthritis Rheum. 2004;50(1):314-326.
- Hymovis [prescribing information]. Parsippany, NJ: Fidia Pharma USA Inc; 2015.
- Smith MM, Russell AK, Schiavinato A, Little CB. A hexadecylamide derivative of hyaluronan (HYMOVIS®) has superior beneficial effects on human osteoarthritic chondrocytes and synoviocytes than unmodified hyaluronan. J Inflamm (Lond). 2013;10:26.
- Finelli I, Chiessi E, Galesso D, Renier D, Paradossi G. A new viscosupplement based on partially hydrophobic hyaluronic acid: a comparative study. Biorheology. 2011;48(5):263-275.
- Bhuanantanondh P, Grecov D, Kwok E. Rheological study of viscosupplements and synovial fluid in patients with osteoarthritis. J Med Biol Eng. 2012;32(1):12-16.
- Henrotin Y, Raman R, Richette P, et al. Consensus statement on viscosupplementation with hyaluronic acid for the management of osteoarthritis. Semin Arthritis Rheum. 2015;45(2):140-149.
- Data on file. Genzyme Corporation.
- Larsen NE, Dursema HD, Pollak CT, Skrabut EM. Clearance kinetics of a hylan-based viscosupplement after intra-articular and intravenous administration in animal models. J Biomed Mater Res B Appl Biomater. 2012;100(2):457-462.
- Boeriu CG, Springer J, Kooy FK, van den Broek LAM, Eggink G. Production methods for hyaluronan. Int J Carbohydr Chem. 2013;2013:1-14.
- Balazs EA, Leshchiner A, Inventors; Biomatrix, Inc, Ridgefield, NJ, assignee. Cross-linked gels of hyaluronic acid and products containing such gels. US patent 4,582,865. April 15, 1986.
- Balazs EA, Leshchiner A, Leshchiner A, Band P, Inventors; Biomatrix, Inc, Ridgefield, NJ, assignee. Chemically modified hyaluronic acid preparation and method of recovery thereof from animal tissues. US patent 4,713,448. December 15, 1987.
- Sanofi. The Making of SYNVISC [video]. 2001.

