SYNVISC® Clinical Studies
Superiority in long-lasting OA knee pain relief demonstrated by head-to-head clinical trials against saline control and other treatments1-6
- This randomized, double-blind, clinical trial assessed the efficacy and safety of three 2-mL injections of SYNVISC given at 1-week intervals in patients with chronic OA of the knee
- Primary endpoints (assessed by patients as measured by VAS [0-100 mm]): Pain during weight-bearing, pain at rest during the night
- In this trial, SYNVISC patients had an average reduction of weight-bearing pain of 48.8% from baseline at 6 months, compared to 25% for saline-treated patients (P=0.005)
- Week 26 mean improvements from baseline for:
- Weight-bearing pain evaluated by patients: 34 mm (SYNVISC) and 19.1 mm (saline) (P=0.005)
- Pain at rest during night evaluated by patients: 24.3 mm (SYNVISC) and 12.8 mm (saline) (P=0.03)
- There were no reports of local adverse reactions to SYNVISC in the injected knee. In clinical trials, the most commonly reported adverse events were transient pain, swelling, and or joint effusion in the injected knee. The following reported adverse events are among those that may occur in association with intra-articular injections, including SYNVISC: arthralgia, joint stiffness, joint effusion, joint swelling, joint warmth, injection site pain, arthritis, arthropathy, and gait disturbance
Pivotal study: Wobig et al.1,3,a
aN=109 (3 weekly, 2-mL injections SYNVISC [n=52] or saline [n=57]). The most commonly reported device-related adverse events (AEs) were transient pain, swelling, and joint effusion in the injected knee.
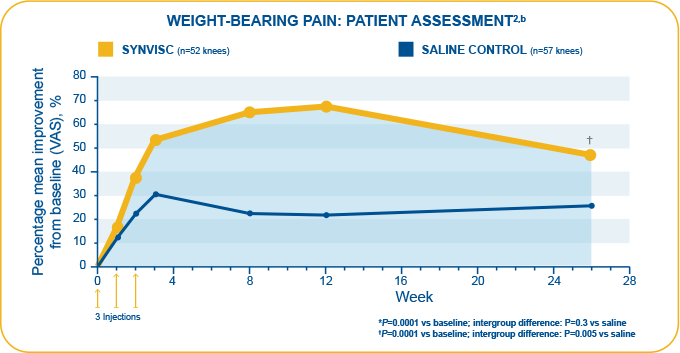
bN=109 (3 weekly 2-mL injections SYNVISC [n=52] or saline [n=57]). Week 26 mean improvement from baseline for weight-bearing pain evaluated by patients: 34 mm (SYNVISC) and 19.1 mm (saline).2 Week 26 data based on patient telephone interviews.
- This randomized, double-blind, placebo-controlled clinical trial assessed the efficacy and safety of one 6-mL injection of Synvisc-One compared to 6 mL of phosphate-buffered saline in patients with chronic OA of the knee
- Primary endpoint: Change from baseline over 26 weeks in WOMAC A (pain) score using 5-point Likert scale where 0=no pain and 4=extreme pain
- In this trial, Synvisc-One patients had a statistically significant change in WOMAC A pain score over 6 months compared with saline-treated patients (36% versus 29%, respectively, P=0.047; ITT population)
- Week 26 mean improvements from baseline for WOMAC A pain scores: -0.84 (Synvisc-One) and -0.69 (saline)
- The incidence of AEs was similar in the 2 groups (Synvisc-One, n=70, 56.9%; saline, n=79, 60.8%).The most commonly reported adverse events were arthralgia, arthritis, arthropathy, injection site pain, and joint effusion. The following reported adverse events are among those that may occur in association with intra-articular injections, including Synvisc-One: arthralgia, joint stiffness, joint effusion, joint swelling, joint warmth, injection site pain, arthritis, arthropathy, and gait disturbance
Pivotal study: Chevalier et al.2,4,b
bN=253 (6 mL of Synvisc-One® + arthrocentesis=124; 6 mL of phosphate-buffered saline placebo + arthrocentesis=129). The most commonly reported device-related AEs were pain in the target knee (coded as “arthralgia”), joint stiffness, joint effusion, arthritis, arthropathy, injection site pain, and joint swelling.
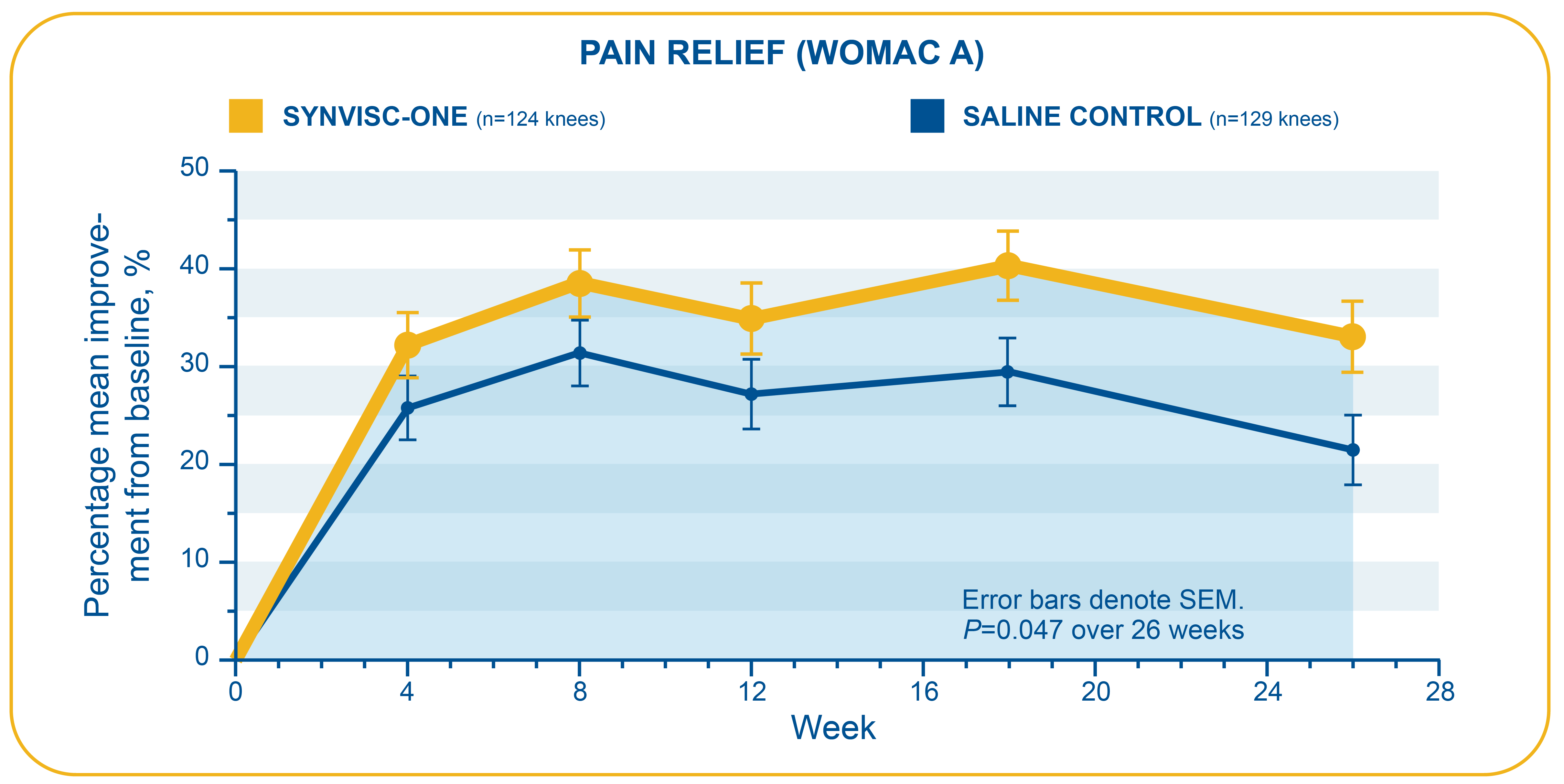
WOMAC=Western Ontario & McMaster Universities Osteoarthritis Index. WOMAC is an instrument used to assess changes in OA symptoms, with each question graded on a 5-point scale (0=low, 4=high).
- This difference was observed as early as 6 weeks (SYNVISC, 3.6 cm; Hyalgan, 0.9 cm) and until 12 months5
- A head to head trial conducted independently of industry compared clinical effectiveness following intra-articular injection with hylan G-F 20 or sodium hyaluronate in patients with symptomatic primary OA of the knee
- Primary endpoint: Intergroup difference in knee pain as measured by VAS at 6 months as reported by the unblinded patient on a 10-cm VAS (0-10, 10 as worst pain)
- In a head-to-head clinical trial conducted independently of industry, SYNVISC provided statistically significantly greater reduction of knee pain from baseline compared to Hyalgan as measured by VAS at 6 months (P=0.02)
- Week 26 mean improvement from baseline for knee pain: 2.5 cm difference in VAS score between SYNVISC and sodium hyaluronate groups (deduced from values represented in Figure 1)
- Secondary endpoint: Week 52 SYNVISC group mean improvement from baseline for knee pain: 3.0 cm
- Study limitations: The number of injections in each group (3 for SYNVISC, 5 for Hyalgan) is a source of treatment bias. Additionally, 2 saline injections in the SYNVISC group were not included due to unknown effect on final outcome. Thus, patients were not blinded to the treatment. Preferred management strategy of OA treatment for individual clinicians was not altered. At 6 months and 12 months, respectively, 8.6% and 30% of SYNVISC patients violated study protocol by taking NSAIDs, compared with 18.5% and 54% of Hyalgan patients
- Treatment-related AEs were similar between the 2 groups (SYNVISC, n=39; sodium hyaluronate, n=30). One severe AE (0.5%) including severe pain, moderate effusion, erythema, and swelling was observed in the SYNVISC group. Symptoms resolved completely by 4 weeks with oral NSAID treatment. All minor AEs were related to the treated knee, and no systemic AEs were recorded in either group
In a head-to-head clinical trial conducted independently of industry, SYNVISC provided significantly greater improvement in OA knee pain from baseline versus Hyalgan at 6 months (intergroup difference: 2.5 cm, P=0.02).5,c
SYNVISC provided greater patient satisfaction with treatment versus Hyalgan at 6 months (7.9 vs 5.0 on a VAS, respectively) in a head-to-head study.5
Safety
Treatment-related AEs were similar between the 2 groups (SYNVISC, n=39; sodium hyaluronate, n=30).5
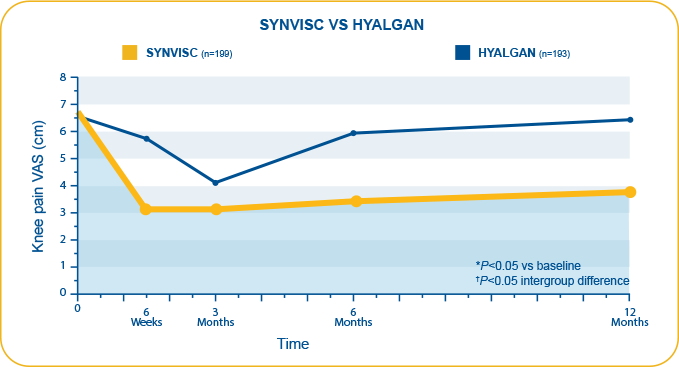
Raman et al.5,c
cN=392 (SYNVISC=199, Hyalgan=193). Study compared clinical effectiveness, functional outcome, and patient satisfaction following intra-articular injection with hylan G-F 20 and sodium hyaluronate in patients with symptomatic primary OA of the knee. Primary endpoint and results: Intergroup difference in knee pain as measured by the unblinded patient on 10-cm VAS (0-10, 10 as worst pain) at 6 months (2.5 cm, deduced from values represented in Figure 1). Secondary endpoints were WOMAC 3.1 (Likert) and Oxford knee scores. Patient satisfaction was quantified on VAS. Safety: All minor AEs were related to the treated knee, and no systemic AEs were recorded in either group. Study limitations: Number of injections in each group (3 for SYNVISC, 5 for Hyalgan) is a source of treatment bias. Additionally, 2 saline injections in the SYNVISC group were not included due to unknown effect on final outcome. Thus, patients were not blinded to the treatment. Preferred management strategy of OA treatment for individual clinicians was not altered. At 6 months, 8.6% of SYNVISC patients violated study protocol by taking NSAIDs, compared with 18.5% of Hyalgan patients.
- Compared with TH, SYNVISC provided significantly greater improvement in OA knee pain at week 12, which was maintained through week 266
- Week 12: (SYNVISC, 0.9 ± 0.1; TH, 0.5 ± 0.1) (P=0.0071)
- Week 26: (SYNVISC, 0.7 ± 0.1; TH, 0.4 ± 0.1) (P=0.0129)
- Patients reporting mild to no pain
- Week 12: 66% for SYNVISC group vs 47% for TH group
- Week 26: 56% for SYNVISC group vs 37% for TH group
- The study was a prospective, multicenter, randomized, single-blind, parallel-group clinical trial. The goal was to compare the efficacy and tolerability of a course of SYNVISC therapy to a typical course of TH therapy
- Primary endpoints: Question A1 of the WOMAC (pain while walking on a flat surface) and VAS scores for patient and investigator (blinded observer) overall assessments
- Compared with long-acting corticosteroid TH in a head-to-head superiority trial, SYNVISC patients showed significantly greater improvement from baseline in pain due to walking on a flat surface (WOMAC A1) than patients in the TH group at 26 weeks (P=0.0129)
- In patient overall assessment VAS pain scores, SYNVISC patients showed an average improvement from baseline of 41% at 26 weeks, compared to 18% for TH-treated patients (P=0.0001)
- Week 26 mean improvements from baseline for:
- WOMAC A1: 0.7 ± 0.1 (SYNVISC) and 0.4 ± 0.1 (TH)(P=0.0129)
- Patient VAS: 28.0 ± 2.5 mm (SYNVISC) and 12.4 ± 2.6mm (TH)(P=0.0001)
- Investigator VAS: 30.0 ± 2.3 mm (SYNVISC) and 18.2 ± 2.5 mm (TH)(P=0.0004)
- Treatment-related AEs were similar between treatment groups (SYNVISC, 21%; TH, 14%). Adverse events were reported in ≥5% of patient treated with SYNVISC or TH and included arthralgia, headache not otherwise specified (NOS), swelling NOS, joint swelling, injection site pain, joint stiffness, injection site swelling, injection site edema, back pain, and joint stiffness. AEs were reported in ≥5% of patients treated with SYNVISC or TH and included arthralgia, headache NOS, swelling NOS, joint swelling, injection site pain, joint stiffness, injection site swelling, injection site edema, back pain, and joint stiffness. No serious AEs were reported in the SYNVISC group
SYNVISC provided significantly greater, longer-lasting relief of OA knee pain versus a long-acting corticosteroid (TH) in a head-to-head superiority trial.6
Safety
Overall incidence of AEs or of any single AE was similar between the 2 groups. Common AEs included arthralgia, swelling, pain, and stiffness. No serious AEs were reported in the SYNVISC group. (SYNVISC, 21%; TH, 14%).6
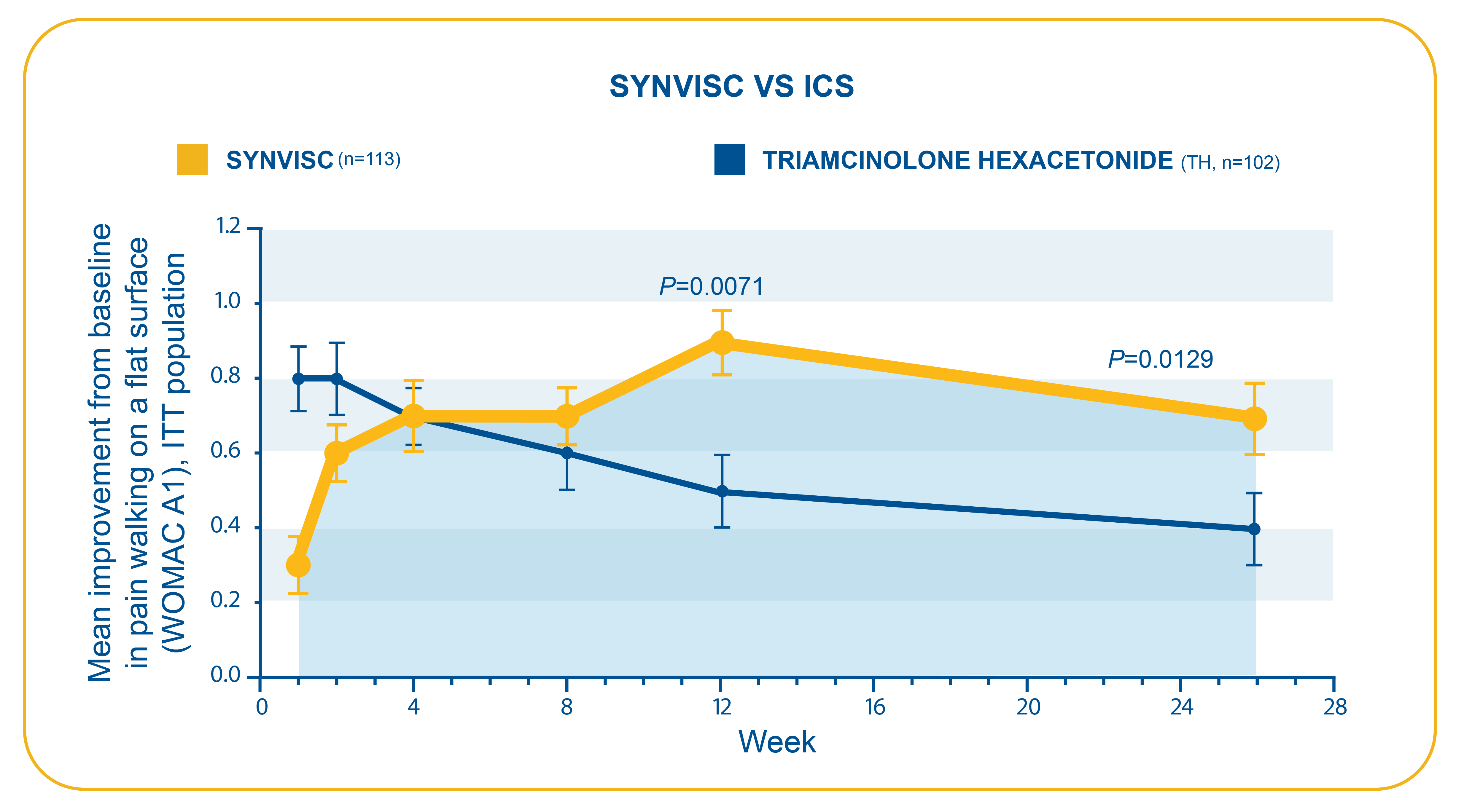
Caborn et al.6,d
dN=218 (SYNVISC=113, TH=105; ITT population). Study was a prospective, multicenter, randomized, single-blind, parallel-group clinical trial. The goal was to compare the efficacy and tolerability of a course of hylan G-F 20 therapy to a typical course of TH therapy. Primary endpoints: Question A1 of the WOMAC (pain while walking on a flat surface).
Resources for Your Patients
MAT-US-2106837-v1.0-07/2021
Indication
SYNVISC® (hylan G-F 20) and SYNVISC-ONE® (hylan G-F 20) are indicated for the treatment of pain in osteoarthritis (OA) of the knee in patients who have failed to respond adequately to conservative nonpharmacologic therapy and simple analgesics, e.g., acetaminophen.
IMPORTANT SAFETY INFORMATION
Important Safety Information
SYNVISC and SYNVISC-ONE are contraindicated in patients with known hypersensitivity to hyaluronan products or patients with infections in or around the target knee. Hypersensitivity reactions including anaphylactic reaction, anaphylactoid reaction, anaphylactic shock and angioedema have been reported for both SYNVISC and SYNVISC-ONE.
Do not concomitantly use disinfectants containing quaternary ammonium salts for skin preparation because hyaluronan can precipitate in their presence. Do not inject SYNVISC or SYNVISC-ONE extra-articularly, into the synovial tissues, into the fat pad or joint capsule, or intravascularly. Some cases of skin necrosis have been reported after intra-articular use of hyaluronic acid. Patients should be instructed to contact their treating physician if signs of skin disorder (such as change of color or open sores) appear.
The safety and efficacy of SYNVISC-ONE in locations other than the knee, or for conditions other than osteoarthritis, or in combination with other intra-articular injectables, or in severely inflamed knee joints have not been established. Use caution when injecting SYNVISC or SYNVISC-ONE in patients allergic to avian proteins, feathers, or egg products; who have evidence of lymphatic or venous stasis in the leg to be treated; or who have severe inflammation in the knee to be treated. Remove any synovial fluid or effusion before injecting SYNVISC or SYNVISC-ONE. Strict adherence to aseptic technique must be followed to avoid joint infection. The safety and effectiveness of SYNVISC and SYNVISC-ONE have not been established in children (≤21 years old) or in pregnant or lactating women. Patients should be advised to avoid strenuous or prolonged weight-bearing activities for approximately 48 hours after treatment.
For SYNVISC
In clinical trials, the most commonly reported adverse events were transient pain, swelling, and/or joint effusion in the injected knee. The following reported adverse events are among those that may occur in association with intra-articular injections, including SYNVISC: arthralgia, joint stiffness, joint effusion, joint swelling, joint warmth, injection site pain, arthritis, arthropathy, and gait disturbance.
View the Complete Prescribing Information for SYNVISC
For SYNVISC-ONE
The most commonly reported adverse events were arthralgia, arthritis, arthropathy, injection site pain, and joint effusion. The following reported adverse events are among those that may occur in association with intra-articular injections, including SYNVISC-ONE: arthralgia, joint stiffness, joint effusion, joint swelling, joint warmth, injection site pain, arthritis, arthropathy, and gait disturbance.
View the Complete Prescribing Information for SYNVISC-ONE
References
- SYNVISC [prescribing information]. Cambridge, MA: Genzyme Corporation; 2014.
- Synvisc-One [prescribing information]. Cambridge, MA: Genzyme Corporation; 2014.
- Wobig M, Dickhut A, Maier R, Vetter G. Viscosupplementation with Hylan G-F 20: a 26-week controlled trial of efficacy and safety in the osteoarthritic knee. Clin Ther. 1998;20(3):410-423.
- Chevalier X, Jerosch J, Goupille P, et al. Single, intra-articular treatment with 6 ml hylan G-F 20 in patients with symptomatic primary osteoarthritis of the knee: a randomised, multicentre, double-blind, placebo controlled trial. Ann Rheum Dis. 2010;69(1):113-119.
- Raman R, Dutta A, Day N, Sharma HK, Shaw CJ, Johnson GV. Efficacy of hylan G-F 20 and sodium hyaluronate in the treatment of osteoarthritis of the knee—a prospective randomized clinical trial. Knee. 2008;15(4):318-324.
- Caborn D, Rush J, Lanzer W, Parenti D, Murray C. A randomized, single-blind comparison of the efficacy and tolerability of hylan G-F 20 and triamcinolone hexacetonide in patients with osteoarthritis of the knee. J Rheumatol. 2004;31(2):333-343.
IMPORTANT SAFETY INFORMATION
Read MoreImportant Safety Information
SYNVISC and SYNVISC-ONE are contraindicated in patients with known hypersensitivity to hyaluronan products or patients with infections in or around the target knee. Hypersensitivity reactions including anaphylactic reaction, anaphylactoid reaction, anaphylactic shock and angioedema have been reported for both SYNVISC and SYNVISC-ONE.
Do not concomitantly use disinfectants containing quaternary ammonium salts for skin preparation because hyaluronan can precipitate in their presence. Do not inject SYNVISC or SYNVISC-ONE extra-articularly, into the synovial tissues, into the fat pad or joint capsule, or intravascularly. Some cases of skin necrosis have been reported after intra-articular use of hyaluronic acid. Patients should be instructed to contact their treating physician if signs of skin disorder (such as change of color or open sores) appear.
The safety and efficacy of SYNVISC-ONE in locations other than the knee, or for conditions other than osteoarthritis, or in combination with other intra-articular injectables, or in severely inflamed knee joints have not been established. Use caution when injecting SYNVISC or SYNVISC-ONE in patients allergic to avian proteins, feathers, or egg products; who have evidence of lymphatic or venous stasis in the leg to be treated; or who have severe inflammation in the knee to be treated. Remove any synovial fluid or effusion before injecting SYNVISC or SYNVISC-ONE. Strict adherence to aseptic technique must be followed to avoid joint infection. The safety and effectiveness of SYNVISC and SYNVISC-ONE have not been established in children (≤21 years old) or in pregnant or lactating women. Patients should be advised to avoid strenuous or prolonged weight-bearing activities for approximately 48 hours after treatment.
For SYNVISC
In clinical trials, the most commonly reported adverse events were transient pain, swelling, and/or joint effusion in the injected knee. The following reported adverse events are among those that may occur in association with intra-articular injections, including SYNVISC: arthralgia, joint stiffness, joint effusion, joint swelling, joint warmth, injection site pain, arthritis, arthropathy, and gait disturbance.
View the Complete Prescribing Information for SYNVISC
For SYNVISC-ONE
The most commonly reported adverse events were arthralgia, arthritis, arthropathy, injection site pain, and joint effusion. The following reported adverse events are among those that may occur in association with intra-articular injections, including SYNVISC-ONE: arthralgia, joint stiffness, joint effusion, joint swelling, joint warmth, injection site pain, arthritis, arthropathy, and gait disturbance.
View the Complete Prescribing Information for SYNVISC-ONE
References
- SYNVISC [prescribing information]. Cambridge, MA: Genzyme Corporation; 2014.
- Synvisc-One [prescribing information]. Cambridge, MA: Genzyme Corporation; 2014.
- Wobig M, Dickhut A, Maier R, Vetter G. Viscosupplementation with Hylan G-F 20: a 26-week controlled trial of efficacy and safety in the osteoarthritic knee. Clin Ther. 1998;20(3):410-423.
- Chevalier X, Jerosch J, Goupille P, et al. Single, intra-articular treatment with 6 ml hylan G-F 20 in patients with symptomatic primary osteoarthritis of the knee: a randomised, multicentre, double-blind, placebo controlled trial. Ann Rheum Dis. 2010;69(1):113-119.
- Raman R, Dutta A, Day N, Sharma HK, Shaw CJ, Johnson GV. Efficacy of hylan G-F 20 and sodium hyaluronate in the treatment of osteoarthritis of the knee—a prospective randomized clinical trial. Knee. 2008;15(4):318-324.
- Caborn D, Rush J, Lanzer W, Parenti D, Murray C. A randomized, single-blind comparison of the efficacy and tolerability of hylan G-F 20 and triamcinolone hexacetonide in patients with osteoarthritis of the knee. J Rheumatol. 2004;31(2):333-343.

